Course of Treatment Agenda
Duration of infusion1,2
Teplizumab is a consecutive 14-day course of treatment. It is administered via IV over 30 minutes, but patients should be advised to plan for at least 2 hours. The time involved includes the pretreatment assessment, flushing the IV access, preparing the medication. Additio The actual infusion time is 60 minutes. The actual infusion time is 60 minutes, including the delivery of pre-medication therapies (30 minutes) and the teplizumab infusion (30 minutes), with the post-therapy observation typically taking 60 minutes. However, additional time is necessary on days that laboratory testing is needed.
It is recommended that the full 14-day treatment be completed without interruption. However, if a dose is missed, it is important that the next dose be given as soon as possible. The patient is not to receive more than one treatment in 24-hours, therefore doubling a dose if one is missed is not recommended.
It is also encouraged that day one of treatment fall on a MONDAY – to ensure that the dose escalations that occur on days 1 thru 5 will occur during the week, when staff are more readily accessible should there be any concerns, problems, etc.
Dosing schedule
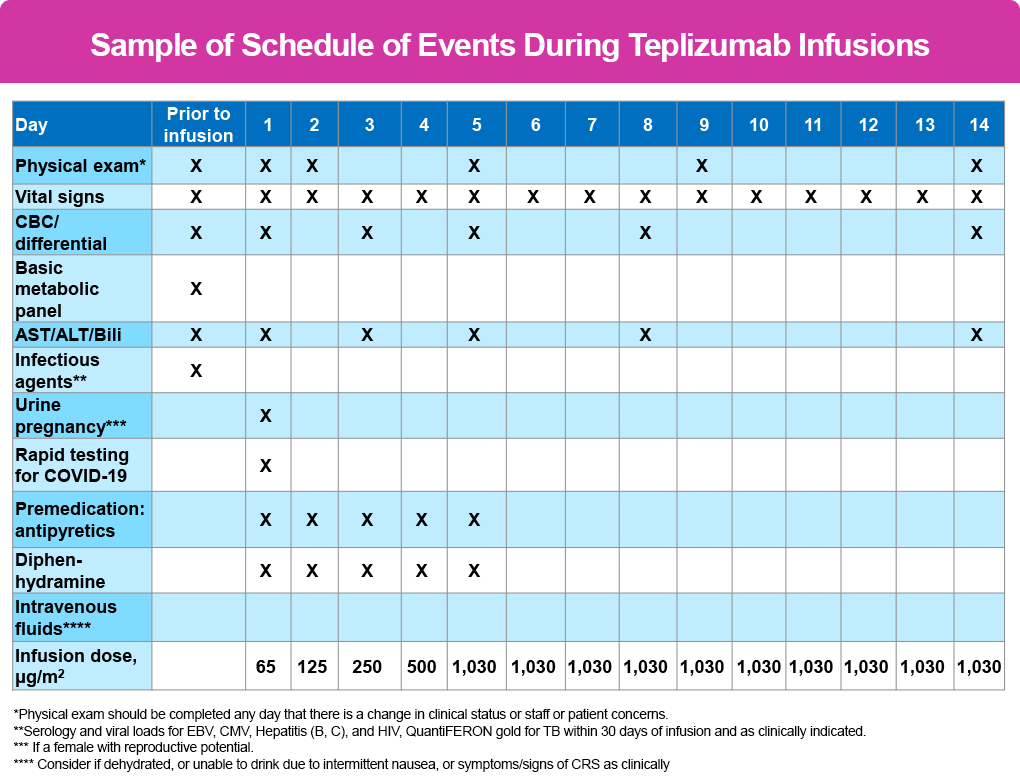
- Day 1: 65 mcg/m2
- Day 2: 125 mcg/m2
- Day 3: 250 mcg/m2
- Day 4: 500 mcg/m2
- Days 5 thru 14: 1030 mcg/m2
Home Supply Order
- Vascular access line care
- Dressing kits
- Adverse drug reaction kit (see section Monitoring for AE)
- Infusion pump
- IV pole with pole clamp
Preparing the infusion (aseptic technique)1,2
- Teplizumab is supplied as a clear, colorless solution in a 2 mg/2 mL, single dose vial (2 vials needed for days 5 thru 14)
- Must remain refrigerated at 36° F to 46° F (2° C to 8° C) – do not freeze
- Must be kept in original packaging to protect from light
- Must be stored upright
- Do not shake the vial
The appropriate dose (see Dosing Schedule above) is to be diluted in 25 mL NS and infusion MUST be completed within 4 hours of dilution. It is to be stored at room temperature (59° F to 86° F or 15° C to 30° C) once diluted. If not administered within 4 hours of preparation, it must be discarded.
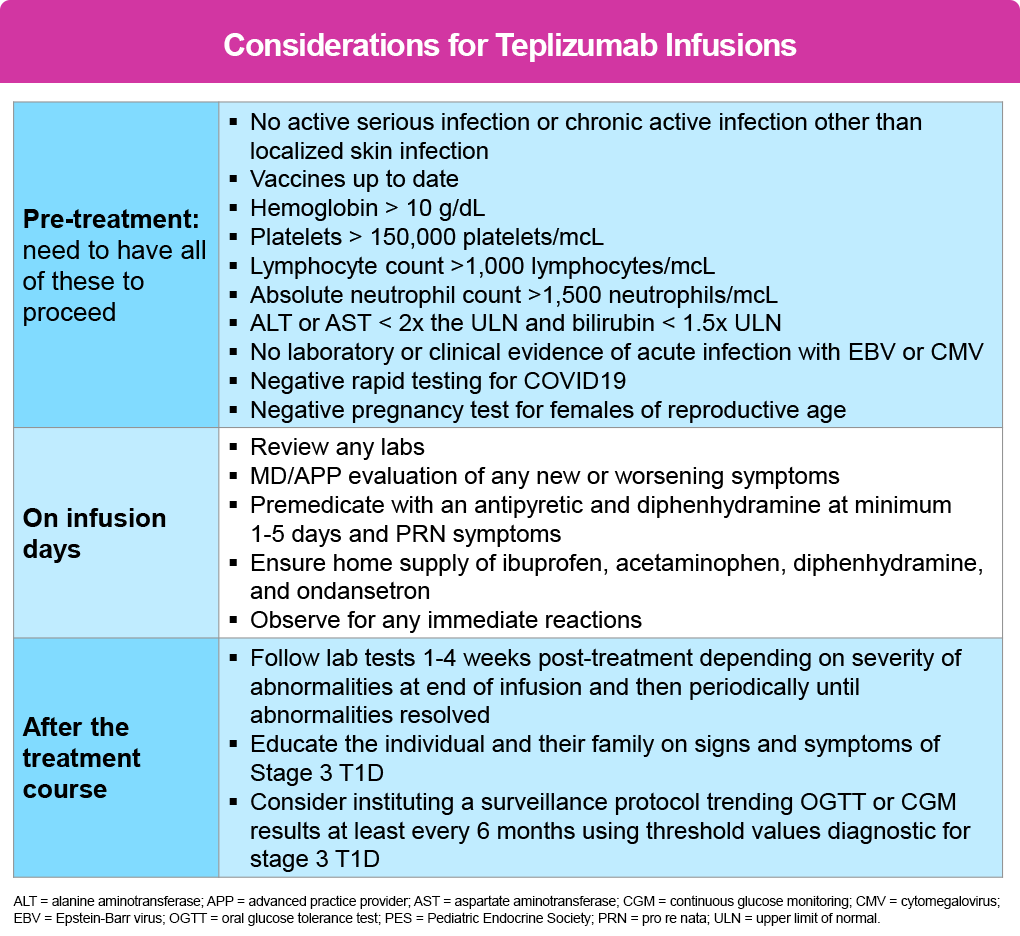
General Assessment & Laboratory Studies (Minimum Recommendations)1,2
- Physical exam – days 1, 2, 5, 9, 14 – and anytime there is a change in clinical status
- Vital signs – performed before, every 15 minutes during and at end of 1 hour post observation for each infusion
CBC, AST/ALT/Bili are all checked prior to infusion therapy, again on days 1, 3, 5, 8, and 14. Many clinicians will have patients obtain labs after the infusion the previous day to help determine the course of action for the following day (ie, labs done after the infusion on day 3 will determine if treatment is given or held on day 4, and so forth).
Pre-medication1,2
Premedication is not only recommended but is part of the instructions in the FDA label and is to be administered on days 1 thru 5 and then as needed on days 6 thru 14.
The purpose of premedication is to reduce the risk for severe CRS, which typically emerges during the initial 5 days of treatment when the doses of teplizumab are escalated. However, the signs and symptoms can occur at any time during the 14-day treatment window as well as up to 28 days after the last infusion.
For pre-medication NSAID (ie, ibuprofen) or acetaminophen (APAP) + antihistamine (ie diphenhydramine) and +/- an antiemetic (ie, ondansetron) are provided to the patient 30 minutes before teplizumab infusion is started.
Some clinicians have found the antiemetic is more helpful later in the day, so it is important to ensure the patient is provided a supply to have on hand at home.
Also, several clinicians have found that using a longer acting antihistamine (ie, loratadine, etc) may be as effective as diphenhydramine, but without the associated drowsiness.
Since elevated liver enzymes are being monitored as a component of CRS, many clinicians have opted for NSAID in place of APAP to reduce the impact on the liver.
Of all the pre-treatment recommendations, adequate hydration is unequivocally the most important to reduce the likelihood of AE. This should be able to be accomplished with oral hydration, but if the patient is unable to consume adequate fluids due to nausea, IV fluids (ie, 500 mL to 1 L of NS) administered either as pre-treatment or post-treatment can be included.
The following tables outline the agenda/schedule of events as recommended by the PES2 and is applicable to all patients regardless of age:
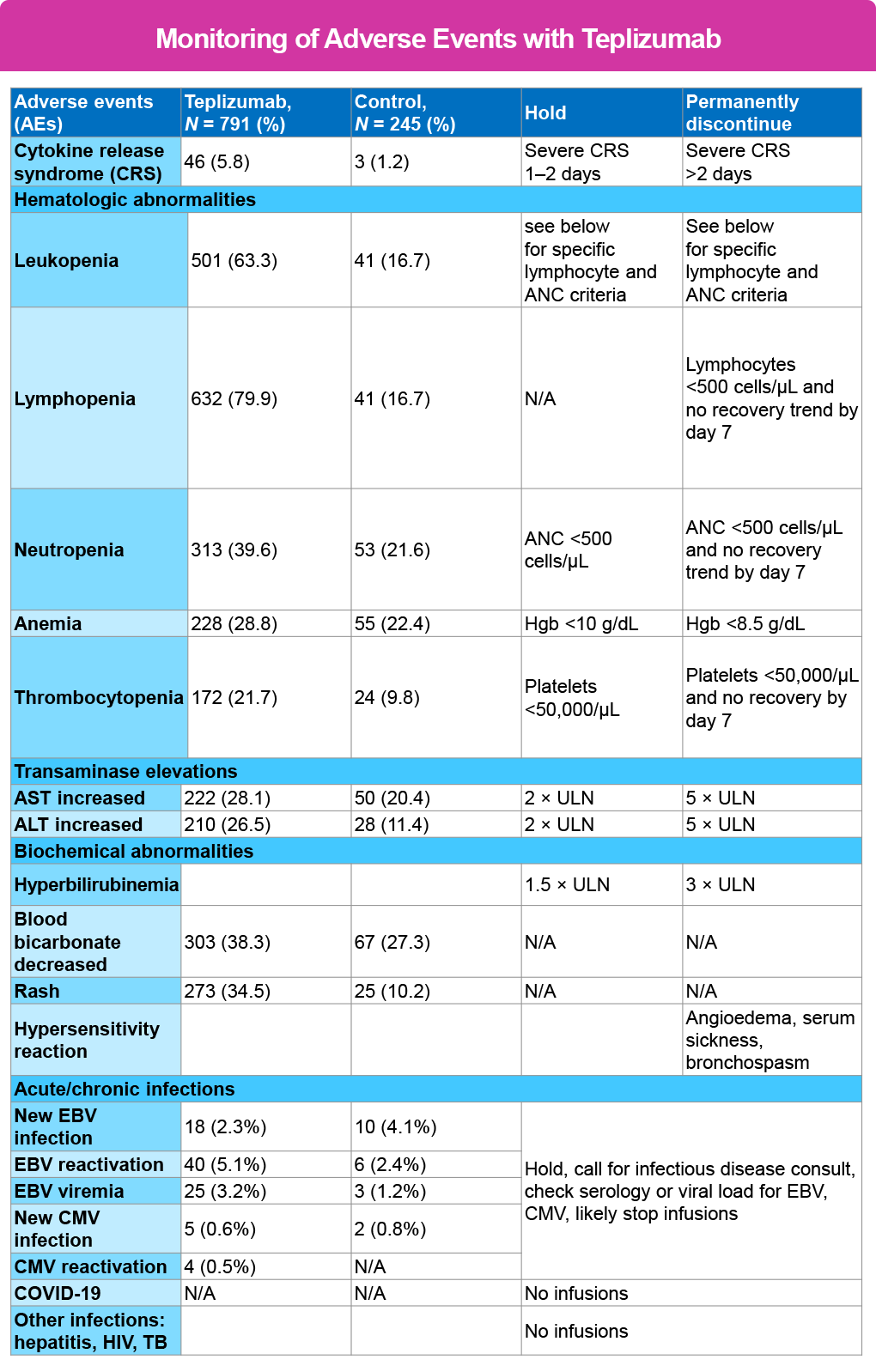
Monitoring for AE1,2
Most of the reactions reported have been mild and able to be mitigated using antipyretics, analgesics, antiemetics, and antihistamines. Therefore, any symptoms experienced by the patient that do not respond to these treatments will warrant prompt emergency treatment.
Fever is the hallmark of CRS, and this may be blunted with NSAID or APAP use. If fever occurs in isolation (without other signs and symptoms of CRS) other acute illnesses or infections must be considered as the cause – especially if a central line is in place.
The most reported reactions have included lymphopenia, elevated liver-associated enzymes (LAE) headache and rash. The lymphopenia has been observed to drop to nadir at approximately day 5 of therapy and then improve with return to baseline by day 42, with several reporting normalization before the completion of therapy.
Patients are instructed to use the utmost caution to prevent exposure to ill contacts for 2 weeks before initiating therapy and until normalization of lymphocytes. This may include wearing a mask when attending school, work, etc.
The rash has been described as maculo-papular, highly pruritic lesions occurring anywhere on the body. The hands and feet are most affected but have also developed on the scalp and other locations. While certainly a nuisance for the patient, all have been shown to resolve spontaneously once teplizumab treatment is completed and without requiring specific treatment. It is important to instruct the patient not to scratch to reduce the risk for a secondary infection. Moisturizers, low dose topical steroids, additional oral antihistamine and topical antihistamines may provide some relief.
Specifically for therapy administered in the patient’s home, it is also highly recommended that the prescription protocol include a home anaphylaxis kit consisting of epinephrine 1 mg/mL (two vials) administered IM per weight-based dosing guide; diphenhydramine 50 mg/mL, 2 mL vial (two vials) administered 25-50 mg IV push, syringes, needles and 0.9% NS flushes (10 mL) to administer.
A standing order stating for the above to be utilized by the clinician in the home in the event of an adverse reaction/anaphylaxis at the same time EMS is activated.
The following table provides guidance for when and duration to either hold or discontinue treatment.2
References
- Tzield® (teplizumab-mzwv). Prescribing information. Provention Bio, Inc; 2023. https://products.sanofi.us/tzield/tzield.pdf
- Mehta S, Ryebets-Lienhard A, Patel N, et al. Pediatric endocrine society statement on considerations for use of teplizumab (TzieldTM) in clinical practice. Horm Res Paediatr. 2024Apr 30:1-12.